GROWTH THROUGH INNOVATION...
Working hours:
Monday to Friday - 9.30 to 6.00 Saturday - 9.30 to 4.00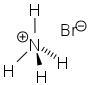
Ammonium Bromide
300 INR/Kilograms
Product Details:
- CAS No 12124-97-9.
- Grade Industrial Grade
- Appearance White Crystalline Powder
- Purity(%) 98%
- Click to view more
X
Ammonium Bromide Price And Quantity
- 25 Kilograms
- 300 INR/Kilograms
Ammonium Bromide Product Specifications
- White Crystalline Powder
- Industrial Grade
- 12124-97-9.
- 98%
Ammonium Bromide Trade Information
- 500 Kilograms Per Week
- 7 Days
- Yes
- Free samples are available
- 25 Kg. HDPE Bags / Drums.
- All India
- ISO CERTIFICATES
Product Description
With strong desire to excel and reach the zenith, we constantly strive to provide optimum quality Ammonium Bromide. This is a white crystalline powder and it starts turning to yellow color because of the oxidation of the traces of Bromide to Bromine when it is exposed to Air. We are working with an aim to develop pure and new chemicals as well as improvise the existing product portfolio. Furthermore, our offered product is used to manufacture following products:
- Photographic films
- Plates & papers
- Corrosion inhibitors in the process of engraving
- Flame retardant products
Ammonium Bromide is a derivative of Bromine. Ammonium Bromide is soluble in water. Ammonium Bromide is a white crystalline powder with a melting point of 452oC. It should be stored in tightly closed containers in a Room Temperature. Ammonium Bromide starts turning to yellow colour because of the oxidation of the traces of Bromide to Bromine when exposed to Air.
Synthesis / Manufacturing Process of Bromides:-
The major raw material of all the Bromides is Elemental Bromine. Bromine was first discovered in 1926. Bromine occurs in the form of Bromides in the Sea Water & in Natural Brine deposits. Bromine brines are treated with Chlorine gas in presence of Air. During this process the Bromide anions are oxidized to Bromine gas by the Chlorine gas. Bromine is further reacted with either Sulphur or Phosphorous &water to give Hydrobromic Acid, which is the intermediate product in manufacturing of various Bromides. Ammonium Bromide is manufactured by taking a reaction between Hydrobromic Acid & Ammonia.
Specifications of Ammonium Bromide:
- Product Name : Ammonium Bromide
- Product Code : B001
- Product Category : Bromides / Iodides
- CAS No. : 12124-97-9
- HSN No. : 28275100
- Synonyms : Ammonium Bromide Pure, Ammonium Bromide Powder
- Molecular Formula : NH4Br
- Molecular Weight : 97.95
- Appearance : White Crystalline Powder
- Solubility : Soluble In Water, Clear Solution
- Assay : 98.0% Min
Uses / Application of Ammonium Bromide:
Ammonium Bromide is used in manufacturing of Photographic Films; Plates & Papers; Corrosion Inhibitors in the process of Engraving; in Lithography;
as a wood preservative & as a Flame retardant.
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese





